Panel of Peers Discussion
The Clinical Trial
Imaging Accuracy Crisis
Breaking studies suggest there is an alarming level of inaccuracy in clinical trial imaging data across leading cancer centers. As all cancer centers face similar challenges, these consistent findings indicate a broader crisis at hand. Join the discussion as a panel of five AACI member cancer centers discuss their own discoveries and what they’re doing to ensure trial imaging data is accurate, compliant, and complete.
Listen to the full panel discussion on:
Error Rates &
Game-Changing Technology
After the implementation of Yunu, we went from 50% inaccurate measurements and we decreased it to 2%! For us, that was a game changer!

Dr. Arcadia Cruz
Associate Administrative Director
UCSD Moore's Cancer Center
Dr. Arcadia Cruz
Associate Administrative Director
UCSD Moore's Cancer Center
Complete Compliance &
Audit Readiness

Kira Pavlik
Associate Director, Clinical Trials Operations
Yale Cancer Center
Kira Pavlik
Associate Director, Clinical Trials Operations
Yale Cancer Center
Collaborative Workflows &
On-Demand Resources
We're creating a platform that you can select anybody you want to have read and they can connect securely and you can invite them into your workflows.

Jeff Sorenson
Co-Founder & CEO
Yunu
Jeff Sorenson
Co-Founder & CEO
Yunu
Delivering a new standard for
clinical trial imaging data and workflow
With thousands of imaging clinical trials relying on the platform each day,
Yunu is serving life science companies and cancer centers to ignite collaboration and accelerate innovation.
Access & Diversity
In oncology, 90% of clinical trials require tumor measurements, but access and diversity issues arise because most trials occur at a few sites. Only 3% of patients from where 80% of the population receives care enroll in trials. To address this, a platform that allows data sharing and collaboration across sites and at the community level is essential."
Dr. Gordon Harris | Co-Dir. of the Tumor Imaging Metrics Core at Dana Farber / Harvard Cancer Center & Co-founder & CSO, Yunu
Collaboration
"We actually end up collaborating a great deal with the radiology department. And once we started looking at the data itself, we realized that more than 50% of all of our findings had some level of issues."
Dr. Arcadia Cruz | Associate Administrative Director UC San Diego Moores Cancer Center
Customer Support
The Yunu team has been incredibly supportive of every issue that we have. We have a really good team. So, it's almost like having an entire partnership that has changed our outcomes. We are meeting the endpoints for our protocols with specificity, efficiency, and reproducibility.
Dr. Arcadia Cruz | Associate Administrative Director UC San Diego Moores Cancer Center
Why choose Yunu?
Why choose Yunu?
Through the power of multi-organization workflows and true cloud-based collaboration, Yunu makes it possible to connect study teams, core labs, sponsors, CROs, and off-site clinical resources so that scaling your clinical trials operation does not distract from busy clinical routines.
4,200+
Clinical trials that extend to
over 8000 clinical sites
2,600+
Registered Yunu solution users
across all types of study staff
400+
Life sciences companies are lead sponsors of trials on Yunu
Standardize
Harmonize
Integrate

Ensure Accuracy. Accelerate Trials.
Ensure Accuracy. Accelerate Trials.
Trial timelines include significant waste created by patchwork solutions to address inaccurate trial imaging data.
If you solve for site data accuracy and workflow at the source, you change the game.


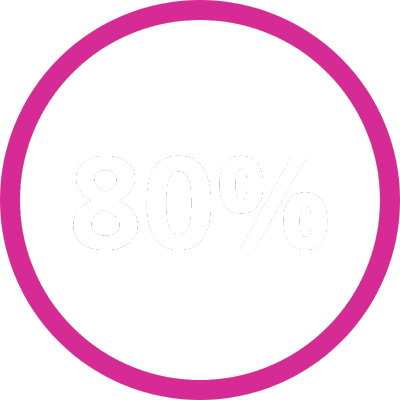
Improve trial team efficiency by 80% and radiologist efficiency by 50%
Security & Compliance

21 CFR Part 11

HIPAA

SOC2 Type II

GDPR

- Connects clinical communities
- Retains trial patients
Ensures accuracy and consistency
- Unifies multi-site trials
A member of the Yunu team will reach out to you shortly!